Medical makers face numerous difficulties in keeping up an ideal clean room
condition, however two agony directs proceed toward present dangers to workers
and dangers to manufacturing processes. In the first place, there is the danger
of employees that handle unsafe drugs, such as, cytotoxics that open laborers
to hurtful work environment situations. As the Medical procedure chain moves from crude synthetic substances stockpiling
to middle exacerbating and into their last and stable medicinal item, the
second issue arises. That is the contamination of clean room situations which
can at last influence the immaculateness and the nature of the manufactured
drug.
Tidy up room defilement is
restricted when the centralization of airborne particles is firmly controlled.
This is practiced by acceptable tidy up room structure, the manner in which the
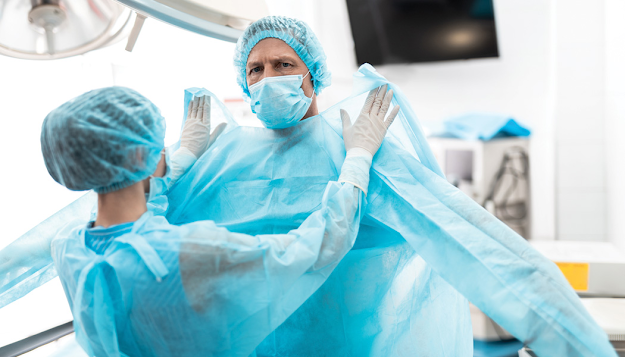
room is cleaned and sterilized, alongside the strategy and sort of administrator
gowning of personal protective equipment.
The influx of contaminated air within pharmaceutical preparing conditions is
for the most part added to the microorganisms and particles that are shed from
the individuals working within the area.
As Per The Institute Of Validation Technology:
"While there are numerous
sources for particle generation, personnel are the main patrons of particles in
clean rooms just as the essential source of microorganisms. Consequently, while
a hazard can emerge from the influx of contaminated air into a clean room this
is less inclined to happen inside an accurately working clean room with viable
air filtration; the more serious hazard is with what befalls particles shed
from individuals working within the clean room when such particles enter the air
stream."
Expanding Pharmaceutical Purity With PPE
Aseptic assembling requests
processes that are liberated from defilement brought about by unsafe
infections, microscopic organisms, or other microorganisms. The pharmaceutical
business faces potential losses of a large number of dollars every year because
of a contaminated pharmaceutical item. Cross-tainting between synthetic
concoctions, outside material moved from workers to process equipment, and biological
contaminants from a representative with messy hands or the beginnings of a
viral infection are only a couple of ways pharmaceuticals are presented to
non-standard impurities.
PPE considerations for the
pharmaceutical business often starts with a discussion of the fabric's ability to
contain contaminants or forestall laborer introduction to the dangers they
present. Be that as it may, theft and solace of substance coveralls are
likewise critical. PPE comfort is a control measure that permits laborers to
remain concentrated on achieving natty gritty errands that request a scope of
development, permitting them to play out these assignments with a more elevated
level of certainty - both of which contribute to better productivity and
quality. Some key design features of PPE for sterile disposable coveralls,
frocks and boot covers include:
- Tunnelized flexible at ankles and wrists for comfort and secure closure.
- Low linting material and no raw edges that can advance airborne fibers.
- Durable material that opposes tears and has solid creases for worker certainty.
- Individually bundled and pre-sanitized to a 10-6 sterility assurance level (SAL).
The triple protection needed between
worker, process, and item requires a fluid and particulate hindrance material
that performs well as the last line of safeguard against cross- contamination.




No comments:
Post a Comment